Tools for Organismal Research
Our goal is to create open-access tools that enable researchers to investigate a range of molecular, organismal, and evolutionary biology questions while maintaining a firm commitment to biological diversity in research organisms.
Behavior
Home Security Cameras for Ectotherm Behavior

Reliably capturing transient animal behavior in the field and laboratory remains a logistical and financial challenge, especially for small ectotherms. We use Wyze camera systems that are affordable, accessible, and suitable to monitor small, cold-blooded animals historically overlooked by commercial camera traps, such as small amphibians.
This setup was published by Goolsby et al, Home security cameras as a tool for behavior observations and science equity. Preprint.
You can find instructions for the setup in multiple languages on our GitHub and instructions on adjusting the focus of Wyze cameras on protocols.io.
A Simple Phototaxis Assay for Aquatic Larvae
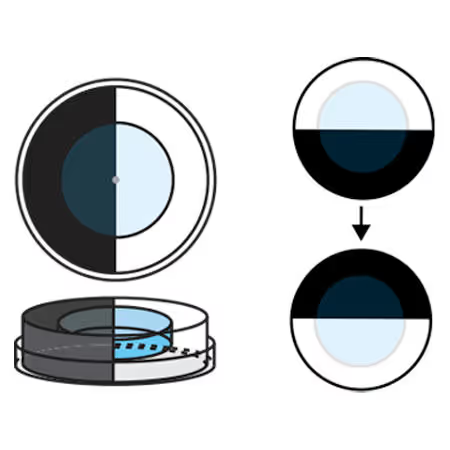
Phototaxis assays are utilized to measure exploratory behaviors and visual capabilities. Here, we detail a simple and low-cost phototaxis assay for aquatic larvae. This assay is useful for simple experiments in laboratory settings and undergraduate teaching laboratories where students can gather data in real-time in a relatively high throughput manner.
We used this assay for tadpoles in the lab (PubMed) and in undergraduate laboratory course (PubMed).
Poison Frog Tadpole Ethogram
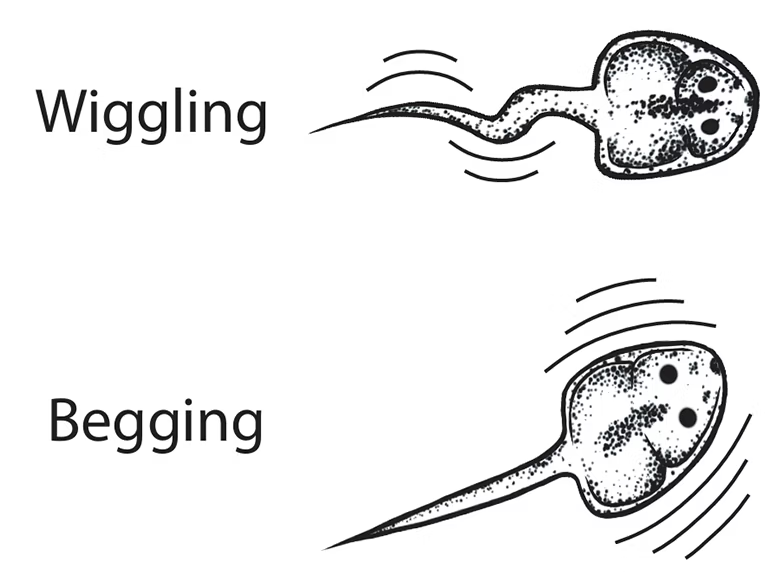
Tadpoles display a rich array of behaviors. This ethogram was developed and illustrated by Dr. Julie Butler and was published in a paper on tadpole sensory behavior (PubMed).
imitatortadethogram_butleretal.pdf (1.41 MB)
Neuroscience
Short heading goes here
We developed a Course Undergraduate Research Experience (CURE) utilizing Caenorhabditis elegans chemotaxis assays to test how natural products are detected by heterospecific nervous systems. These experiments focus on ant-derived compounds and can be conducted in an undergraduate laboratory course, where new insights into interspecies interactions can be generated through genuine research experiences in a classroom setting. This assay is used in BIO161: Organismal Biology Lab and has resulted in several peer-reviewed publications with all students as co-authors. PMIDs: 38596360, 37008729, 32550506 Protocol
Multiplex RNAscope for Frog Brain Tissue
In neuroscience, different types of cells need to be labeled in brain tissue to show us which cells have been recently active and/or to tell us what kind of cells they are and where they are located. Sometimes this is done with antibodies, but finding specific antibodies that work for non-mammalian research organisms can be challenging. We often use RNAscope to label the mRNA of specific genes, enabling us to identify cell types and other genes of interest. Here we detail our optimized protocol for using RNAscope for frog brain tissue.
.avif)
Functional Genomics
Short heading goes here
We developed a Course Undergraduate Research Experience (CURE) utilizing Caenorhabditis elegans chemotaxis assays to test how natural products are detected by heterospecific nervous systems. These experiments focus on ant-derived compounds and can be conducted in an undergraduate laboratory course, where new insights into interspecies interactions can be generated through genuine research experiences in a classroom setting. This assay is used in BIO161: Organismal Biology Lab and has resulted in several peer-reviewed publications with all students as co-authors. PMIDs: 38596360, 37008729, 32550506 Protocol
Tissue-Specific Expression of Genes from Plasmid DNA
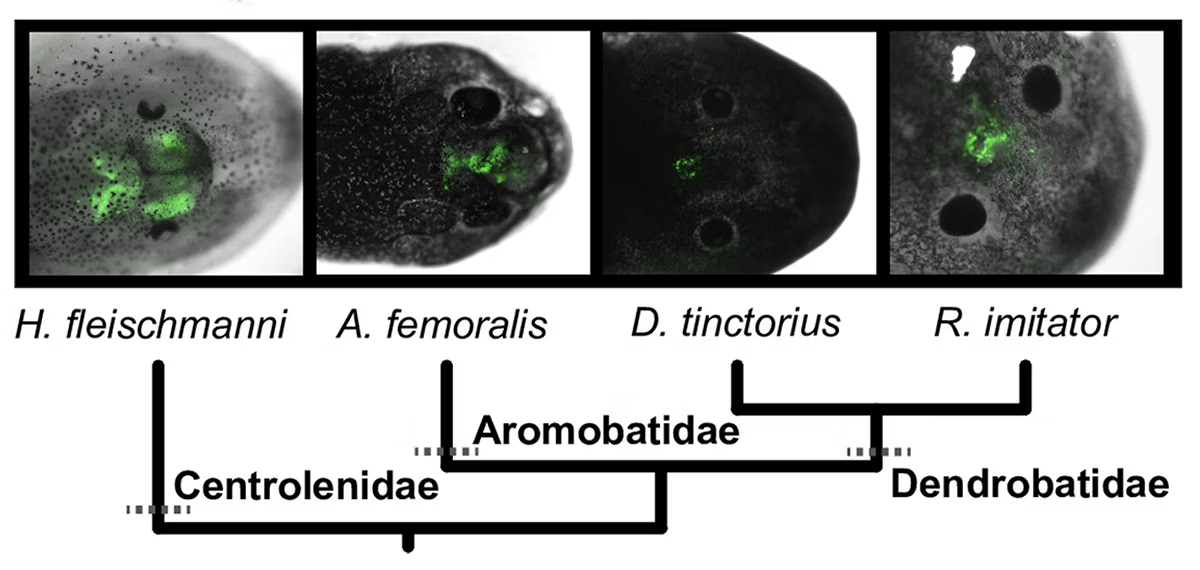
Expressing exogenous genes with temporal and spatial specificity is challenging with unusual research organisms. Here, we detail how to express exogenous genes from the electroporation of plasmids into the brain and muscle of poison frog tadpoles. This protocol is useful for expressing fluorescent reporters and other genes of interest encoded on plasmids. This method has been published (PubMed).
Using Morpholinos to Knockdown Proteins
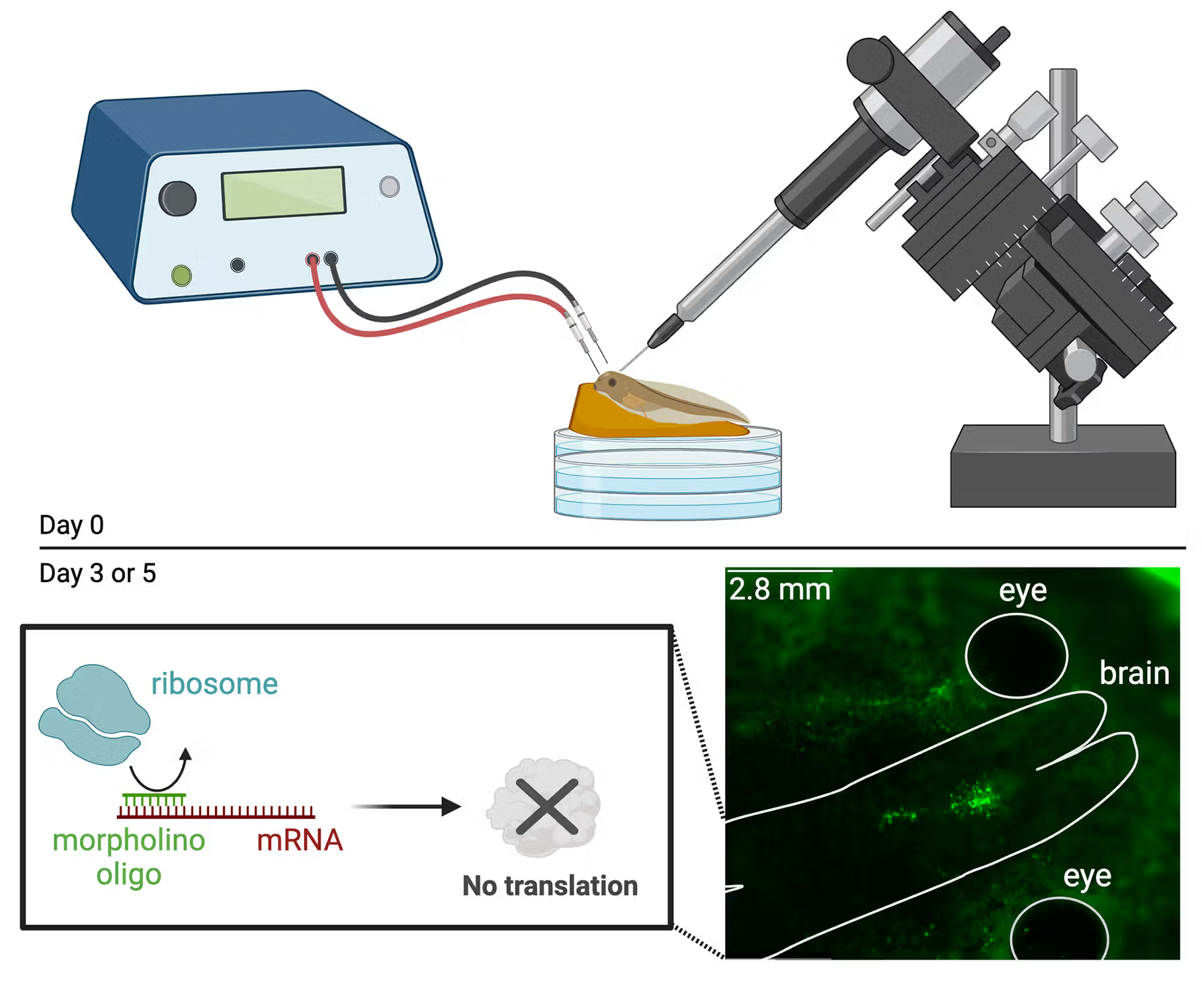
Knocking down the abundance of proteins of interest is important for functionally testing their role in biological processes. Here, we detail how to use morpholinos to knock down protein abundance in tadpole brain tissue. We also present a relatively inexpensive semi-quantitive dot blot method for assessing protein knockdown. While this manuscript is currently in revision, the protocol is available on protocols.io
Frog Genomes
Short heading goes here
We developed a Course Undergraduate Research Experience (CURE) utilizing Caenorhabditis elegans chemotaxis assays to test how natural products are detected by heterospecific nervous systems. These experiments focus on ant-derived compounds and can be conducted in an undergraduate laboratory course, where new insights into interspecies interactions can be generated through genuine research experiences in a classroom setting. This assay is used in BIO161: Organismal Biology Lab and has resulted in several peer-reviewed publications with all students as co-authors. PMIDs: 38596360, 37008729, 32550506 Protocol
Mimetic Poison Frog (Ranitomeya imitator)
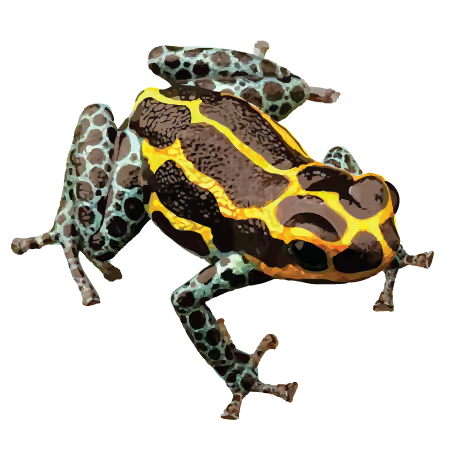
Behavior: biparental and monogamous adults, begging and aggressive tadpoles
Physiology: chemically defended, Müllarian mimic
Access: NCBI Genome Assembly GCF_032444005.1
Publication: in progress - coming in concert with the evolution of monogamy study
Brilliant Thighed Poison Frog (Allobates femoralis)

Behavior: flexible parental care, territorial males, social tadpoles
Physiology: not chemically defended in nature
Access: NCBI Genome Assembly ASM3357653v1
Publication: writing in progress.
Note: The genome has premature stop codons for many genes, making annotation difficult. This genome is being resequenced to address these shortcomings.
Variable Poison Frog (Ranitomeya variabilis)

Behavior: male parental care, aggressive tadpoles
Physiology: chemically defended, polymorphic coloration
Access: NCBI Genome Assembly GCF_051348905.1
Publication: In progress - will be published in concert with the evolution of monogamy study
Golden Poison Frog (Phyllobates terribilis)

Diablito Poison Frog (Oophaga sylvatica)

Behavior: female parental care, begging tadpoles, territorial males
Physiology: chemically defended, polymorphic coloration
Access: NCBI Genome Assembly ASM3357655v1
Publication: writing in progress
Other Animal Genomes
Short heading goes here
We developed a Course Undergraduate Research Experience (CURE) utilizing Caenorhabditis elegans chemotaxis assays to test how natural products are detected by heterospecific nervous systems. These experiments focus on ant-derived compounds and can be conducted in an undergraduate laboratory course, where new insights into interspecies interactions can be generated through genuine research experiences in a classroom setting. This assay is used in BIO161: Organismal Biology Lab and has resulted in several peer-reviewed publications with all students as co-authors. PMIDs: 38596360, 37008729, 32550506 Protocol
Wolf Spider (Hogna lenta)
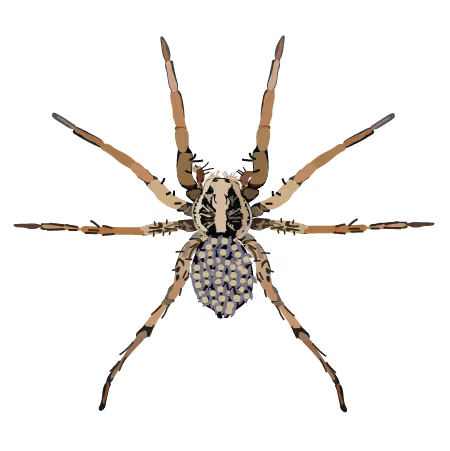
Behavior: burrowing, female parental care
Physiology: moms do not eat during parental care
Status: complete, upload to NCBI will start soon
Publication: coming soon with study on parental care
Common Blue Tongue Skink (Tiliqua scincoides)
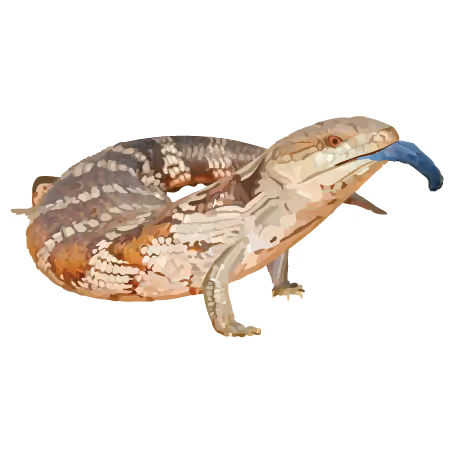
Behavior: polygamous, long-lived
Physiology: live-bearing, blue tongue
Access: NCBI Genome Assembly GCF_035046505.1
Publication: in progress
Note: Feedback from the scientific community has noted this assembly lacks micro-chromosomes typical of this genus.
Blue-Breasted Quail (Excalfactoria chinensis)

Behavior: monogamous
Physiology: polymorphic coloration, used in aviculture
Access: NCBI Genome Assembly GCF_039877785.1
Publication: in progress - to be published in concert with the evolution of monogamy study
Cheveron Butterflyfish (Chaetodon trifascialis)

Behavior: polygamous, no parental care
Physiology: corallivore
Access: NCBI Genome Assembly GCF_039877785.1
Publication: in progress - to be published in concert with the evolution of monogamy study
Threadfin Butterflyfish (Chaetodon auriga)

Behavior: monogamous, no parental care
Physiology: corallivore
Access: NCBI Genome Assembly GCF_051107435.1
Publication: in progress - to be published with evolution of monogamy study
Other Resources
Short heading goes here
We developed a Course Undergraduate Research Experience (CURE) utilizing Caenorhabditis elegans chemotaxis assays to test how natural products are detected by heterospecific nervous systems. These experiments focus on ant-derived compounds and can be conducted in an undergraduate laboratory course, where new insights into interspecies interactions can be generated through genuine research experiences in a classroom setting. This assay is used in BIO161: Organismal Biology Lab and has resulted in several peer-reviewed publications with all students as co-authors. PMIDs: 38596360, 37008729, 32550506 Protocol
Poison Frog Color Palette

A collection of color palletes inspired by Neotropical poison frogs. With more than 200 brightly colored species, Neotropical poison frogs paint the rain forest in vivid hues that shout a clear message: "I'm toxic". Spice up your plots with poison frogs and give your dataviz a toxic twist. But wait, we also included some color palettes inspried by other pretty frog species, because.... why not?
Chemical Ecology in the Classroom
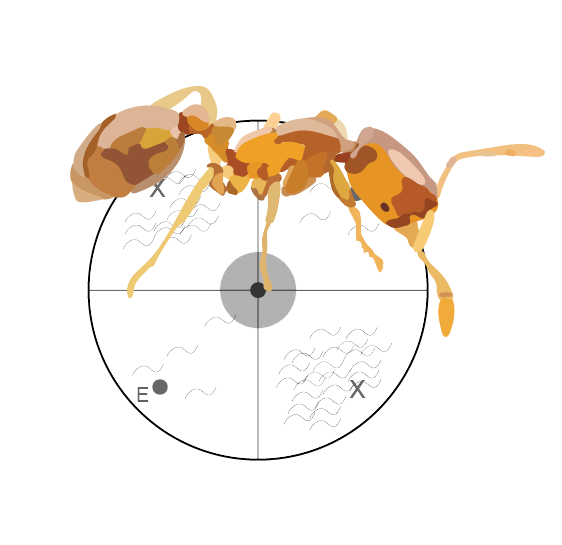
We developed a Course Undergraduate Research Experience (CURE) utilizing Caenorhabditis elegans chemotaxis assays to test how natural products are detected by heterospecific nervous systems. These experiments focus on ant-derived compounds and can be conducted in an undergraduate laboratory course, where new insights into interspecies interactions can be generated through genuine research experiences in a classroom setting.
This assay is used in BIO161: Organismal Biology Lab and has resulted in several peer-reviewed publications with all students as co-authors. PMIDs: 38596360, 37008729, 32550506
Growing up Frog Skin Microbes

Staging Guide for Poison Frog Tadpoles

Staging guide for Ranitomeya imitator tadpoles. Published as supplementary materials in a paper on tadpole sensory development (PubMed).
Rimi_tadpole_stages.pdf (1.14 MB)
Non-Lethal 3-D Imaging Techniques for Estimating Tadpole Morphology

Body mass and morphology are important measurements for physiology and evolution studies. Here we detail a method for obtaining 3D images and morphology measurements in aquatic larvae. We also include how to create a body mass estimation method to reduce animal experimentation numbers.

Accessible Science

.avif)
Innovative Research






